Assessment of protolytic properties of soil organic polyelectrolytes by pK-spectroscopy
DOI:
https://doi.org/10.31251/pos.v8i1.299Keywords:
acid-base properties; titration; pK-spectrum; soil; fulvic acids; water-soluble organic matter.Abstract
The aim of the study was to test the possibility of using the pK spectroscopy to evaluate the acid-base properties of humic substances and water-soluble soil fractions.
Location and time of the study. The studied preparations of fulvic acids were isolated from the organogenic horizons of peaty-podzolic surface-gley soil (Eutric Albic Stagnic Histic Retisol (Loamic)), virgin and arable podzolic soils (Eutric Albic Retisols (Loamic)). The soils are located in the middle taiga zone (Maximovsky station of the Institute of Biology, 8 km west of Syktyvkar and the field of Syktyvkar state farm, 5 km south-west of Syktyvkar). Soils were sampled from 1 to 30 August 2014. To study the protolytic properties of the soil liquid phase, samples of the organogenic horizons of three soils were also taken in July 2018: sod-podzolic non-gleyed (Eutric Albic Retisol (Loamic)), sod-podzolic gleyed (Distric Gleyic Retisol (Loamic)) and sod-podzolic gleyed (Eutric Albic Retisol). The soils are located 1 km north-west of Krutotyla village, Priluzsky district (Komi Republic, Russia).
Methods. Potentiometric titration of soil organic polyelectrolyte solutions was carried out at 25±1 °C. The EMF of the titrated solutions was measured on a pH-150 ionometer using an ESL-15-11 glass electrode. Aliquots of aqueous solutions of fulvic acids and soil extracts were titrated with HCl or NaOH solutions. Titration was carried out in pH ranges from the starting point of titration to 3.0 when titrating with acid solution and to 10.0 when titrating with base solution. Titration of solutions of organic compounds was carried out in 3–5 replicates. The pK-spectra of the systems were calculated from the potentiometric titration curves using the “PKSVD” computer program.
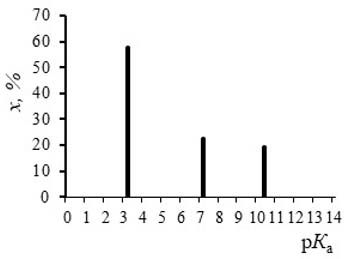
Results. The results of the pK-spectroscopy testing showed that this method was more illustrative and informative than the traditional method of continuous potentiometric titration. The “PKSVD” programme allows pK-spectra to be calculated with sufficient accuracy for complex natural polyelectrolytes such as fulvic acids and water-soluble organic substances of soils. The use of the “SPLINE” programme for experimental relathinship smoothing allows the results of pK-spectra construction to be improved. The application of the pK-spectroscopy method allowed to determine the values of acidity constants for groups of complex natural systems, to calculate the number of acid groups and to obtain new results of both fundamental and applied character. The organic polyelectrolytes of the soils studied revealed the presence of three to five ionogenic groups with pK values ranging from 3.2 to 9.6.
Conclusions. The method of pK-spectroscopy using linear regression analysis with restrictions on non-negativity of solutions opens wide possibilities for potentiometric analysis of complex protolytic systems, including various environmental objects. This method significantly extends the understanding of the physico-chemical properties of humic substances and soil organic matter.
Downloads
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 The Journal of Soils and Environment

This work is licensed under a Creative Commons Attribution 4.0 International License.






